
Given that is was infant formula, you would think that the FDA and CDC investigators would be updating the public more often on the size and scope of the outbreak and if a cause for the outbreak has been found (ingredient or production contamination).
Setting aside that recall product was still being found on store shelves as of a few days ago, all families need to know what the “Root Cause” of this outbreak was to prevent a next outbreak and perhaps allow ByHeart to reenter the market. Not knowing the most likely cause of the outbreak is not acceptable and would certainly be a reason to alert the public on the risks of infant formula in the U.S., and in particular, ByHeart, if it ever is allowed to restart production.
The FDA and CDC, in collaboration with the California Department of Public Health (CDPH), Infant Botulism Treatment and Prevention Program (IBTPP), and other state and local partners, continue to investigate a multistate outbreak of infant botulism. Epidemiologic and laboratory data show that ByHeart Whole Nutrition infant formula might be contaminated with Clostridium botulinum, which is causing infant illness in multiple regions of the country.
ByHeart’s and FDA’s investigations into the root cause of the outbreak are ongoing, and at this time, FDA cannot rule out the possibility that contamination might have affected all ByHeart formula products. In response, CDC broadened the case definition to include any infant with botulism who was exposed to ByHeart formula at any time since the product’s release in March 2022. As of December 10, 2025, a total of 51 infants with suspected or confirmed infant botulism and confirmed exposure to ByHeart Whole Nutrition infant formula (various lots) have been reported from 19 states.
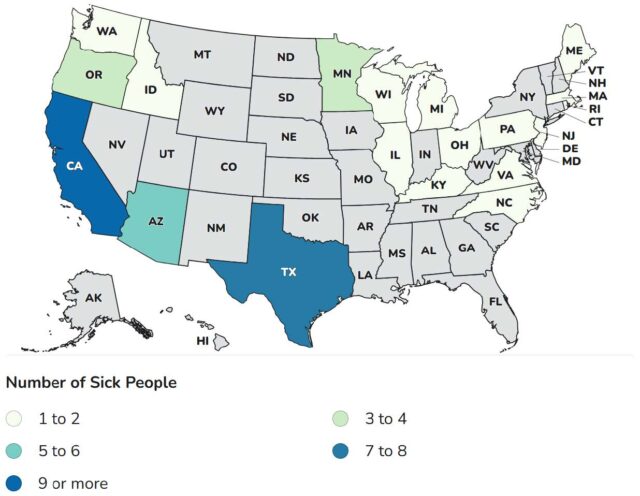
States with illnesses: Arizona 5, California 12, Idaho 2, Illinois 2, Kentucky 1, Massachusetts 2, Maine 1, Michigan 1, Minnesota 3, North Carolina 2, New Jersey 1, Ohio 1, Oregon 4, Pennsylvania 1, Rhode Island 1, Texas 8, Virginia 1, Washington 2 and Wisconsin 1.
Previously, case counts included illnesses from August 1, 2025, onward. With the expanded definition, CDC and state partners identified 10 additional cases that occurred from December 2023 through July 2025. At this time, no cases have been identified between March 2022 and December 2023. All 10 are confirmed infant botulism cases with documented exposure to ByHeart formula.
Laboratory confirmation for some cases is ongoing. Illnesses started on dates ranging from December 24, 2023 to December 1, 2025. All 51 infants were hospitalized. No deaths have been reported to date. The infants range in age from 16 to 264 days and 22 (43%) are female.